how many neutrons does bromine have|How to find the Number of Protons, Electrons, Neutrons for : Baguio Bromine is a chemical element with atomic number 35 and atomic mass 79.904 u. It has two stable isotopes with 79 and 81 neutrons each. But what you and I don't instantly get about Karraminah Clarisse Del Rosario (or @kcdelro as you might know her) is that she operates on another stratosphere, and you can't try to define who or what she is. Between hopping over the Atlantic from New York to London and gallivanting in her native Manila, .
PH0 · How to find the Number of Protons, Electrons, Neutrons for
PH1 · How Many Protons, Neutrons and Electrons Does Bromine Have?
PH2 · Chemical Elements.com
PH3 · Bromine (Br)
PH4 · Bromine
PH5 · 2.3: Atomic Structure and Symbolism
See Inkyminky's porn videos and official profile, only on Pornhub. Check out the best videos, photos, gifs and playlists from amateur model Inkyminky. Browse through the content she uploaded herself on her verified profile. Pornhub's amateur model community is here to please your kinkiest fantasies.Penjelasan: Layanan Skor pertandingan sepak bola langsung di Livescore.in menampilkan skor pertandingan sepak bola secara langsung lebih dari 500 liga sepak bola, kejuaraan dan turnamen (misalnya Liga Super, Liga Primer Inggris dan La Liga), menampilkan juga klasemen liga, pencetak gol, skor turun minum, kartu kuning/merah .
how many neutrons does bromine have*******BlockElements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Atomic numberThe number of protons in an . Bromine is a chemical element with atomic number 35 and atomic mass 79.904 u. It has two stable isotopes with 79 and 81 neutrons each. Bromine - Protons - Neutrons - Electrons - Electron Configuration. Bromine has 35 protons and electrons in its structure. The total number of neutrons in the nucleus of an atom is called the neutron .
In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Bromine (Br). From the Periodi.Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine has thirty .Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between .
Symbol: Br. Atomic Number: 35. Atomic Mass: 79.904 amu. Melting Point: -7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35. Number of Neutrons: 45. .how many neutrons does bromine have How to find the Number of Protons, Electrons, Neutrons for Bromine is a chemical element of the periodic table with chemical symbol Br and atomic number 35 with an atomic weight of 79.901 u and is classed as nonmetal and is part of .
For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. .How to find the Number of Protons, Electrons, Neutrons for For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. .
Bromine (35 Br) has two stable isotopes, 79 Br and 81 Br, and 35 known radioisotopes, the most stable of which is 77 Br, with a half-life of 57.036 hours.. Like the radioactive isotopes of iodine, radioisotopes of bromine, collectively radiobromine, can be used to label biomolecules for nuclear medicine; for example, the positron emitters 75 Br and 76 Br .

How many protons and neutrons does it contain, and what is its charge? [reveal-answer q=”488361″]Show Answer[/reveal-answer] . Bromine has two isotopes 79 Br and 81 Br, whose masses (78.9183 and 80.9163 . Protons. A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one ( + 1) and a mass of 1 atomic mass unit (amu), which is about 1.67 × 10 − 27 kilograms. Bromine has two naturally occurring isotopes: bromine-79 has a mass of 78.9183 u and an abundance of 50.69%, and bromine-81 has a mass of 80.92 u and an abundance of 49.31%. The equation above can be used to solve for the relative atomic mass of bromine: atomic mass of Br = (0.5069 x 78.9183 u) + (0.4931 x 80.92 u) = 79.91 u
Name of the isotope: Bromine-81; Br-81 Symbol: 81 Br or 8135 Br Mass number A: 81 (= number of nucleons) Atomic number Z: 35 (= number of protons) Neutrons N: 46 Isotopic mass: 80.91690 (9) u ( atomic weight of Bromine-81) Nuclide mass: 80.8977004 u (calculated nuclear mass without electrons) Mass excess: -77.40715 MeV Mass defect: .
When the numbers of these subatomic particles are not equal, the atom is electrically charged and is called an ion. The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons.
An atomic mass unit is defined as a mass equal to one twelfth of an atom of carbon-12. The mass of any isotope of any element is expressed in relation to the carbon-12 standard. For example, one atom of helium-4 has a mass of 4.0026amu 4.0026 amu. An atom of sulfur-32 has a mass of 31.972 amu 31.972 amu. Br-80 has 35 protons, the element has 35 electrons (bromide anion has one more), and 80-35=45 neutrons. Bromine-80 has 35 protons, as all bromine atoms do. The number of neutrons can be calculated .
Bromine's atomic number is 35 and its atomic weight is 80, so it contains 45 neutrons. 80 total particles - 35 protons = 45 neutrons. In a bromide ion there are 45 neutrons. There are also 35 .how many neutrons does bromine have Cobalt has 27 protons, 32 neutrons and 27 electrons: 28: Nickel has 28 protons, 31 neutrons and 28 electrons: 29: Copper has 29 protons, 35 neutrons and 29 electrons: 30: Zinc has 30 protons, 35 .Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure. Element Groups: Alkali Metals Alkaline Earth Metals . Basic Information Name: Bromine Symbol: Br Atomic Number: 35 Atomic .
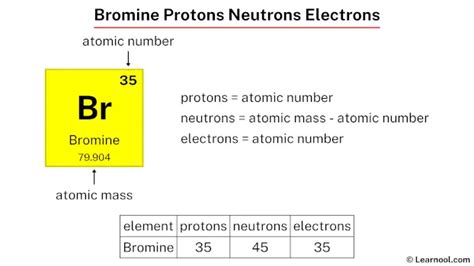
F. Fluorine. 9. 18.998. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. Neutrons = atomic mass – atomic number. Bromine neutrons | Image: Learnool. The atomic mass of bromine is 79.904, so we’ll take the roundup value as 80. And the atomic number of bromine is 35. Subtract the atomic number (35) from the atomic mass (80). Hence, bromine has a total of 80 – 35 = 45 neutrons. Figure 3.4.1 3.4. 1: The social security number subatomic-the proton. Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons. Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.
We would like to show you a description here but the site won’t allow us.
Answer: How many proton, neutrons and electron does bromine have? A bromine atom has 35 protons, 45 neutrons, and 35 electrons.; Explanation: To find each of them: Look at the atomic number to find the number of protons, which is 35, so there are 35 protons in a bromine atom, regardless of the isotope.
1,256 via gonzales scandal FREE videos found on XVIDEOS for this search. Language: Your location: USA Straight. Login Join for FREE Premium. Best Videos; Categories. Porn in your language; 3d; AI; . Colombian influencer Lina Henao fucking with porn actor Max Cartel in a well-known place in Medellin overlooking the public street.
how many neutrons does bromine have|How to find the Number of Protons, Electrons, Neutrons for